Arthur Brain
Well-known member
Then there shouldn't be any difficulty in citing to a credible source then should there?This is called special pleading.
Where information comes from has no bearing on the validity of the information.
Then there shouldn't be any difficulty in citing to a credible source then should there?This is called special pleading.
Where information comes from has no bearing on the validity of the information.
Not to mention that he is so busy whinging that he's missed the cite twice now. :chuckle:This is called special pleading.
Where information comes from has no bearing on the validity of the information.
yesUh, yeah, this entire lnk undermines vaccination...
*facepalm*
No, it doesn't doofus and nor does the entirety of evidence but don't worry, nobody's forcing you to get jabbed.yes
the problem is the vax doesn't work at prevention or spreading
by these numbers the vaxd are no better off than the natural
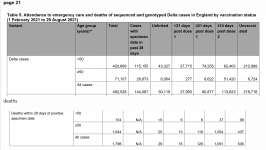
View attachment 1887
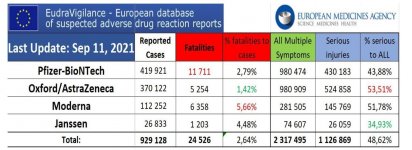
don't forget to factor in the vax serious injuries of 1,126,869 in the EU
.
View attachment 1888
confession through projectionBest stay in the basement then.
Nah, I don't have a basement.confession through projection
I don't have a basement either. I have a small desk in a corner of my living room where I do research over the web. I don't get my positions and narratives from the TV, from politicians, from campaign talking points, or other cheap and weak sources. I research what is posted on the web and try to either prove or disprove what is reported by digging deeper to gather as many facts as possible before drawing conclusions.Nah, I don't have a basement.
I don't have a basement either. I have a small desk in a corner of my living room where I do research over the web. I don't get my positions and narratives from the TV, from politicians, from campaign talking points, or other cheap and weak sources. I research what is posted on the web and try to either prove or disprove what is reported by digging deeper to gather as many facts as possible before drawing conclusions.
Imagine how heinous this is. Our own government is pushing a dangerous vaccine and shutting down all voices of reason and science who are vociferously warning against the vaccine.Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial
link on how not to do a study
But, for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails.
... poor laboratory management, patient safety concerns, and data integrity issues. Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management. Exasperated that Ventavia was not dealing with the problems, Jackson documented several matters late one night, taking photos on her mobile phone. One photo, provided to The BMJ, showed needles discarded in a plastic biohazard bag instead of a sharps container box. Another showed vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants. Ventavia executives later questioned Jackson for taking the photos.
...
In a recording of a meeting in late September2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. “In my mind, it’s something new every day,” a Ventavia executive says. “We know that it’s significant.”
h**ps://www.bmj.com/content/375/bmj.n2635
